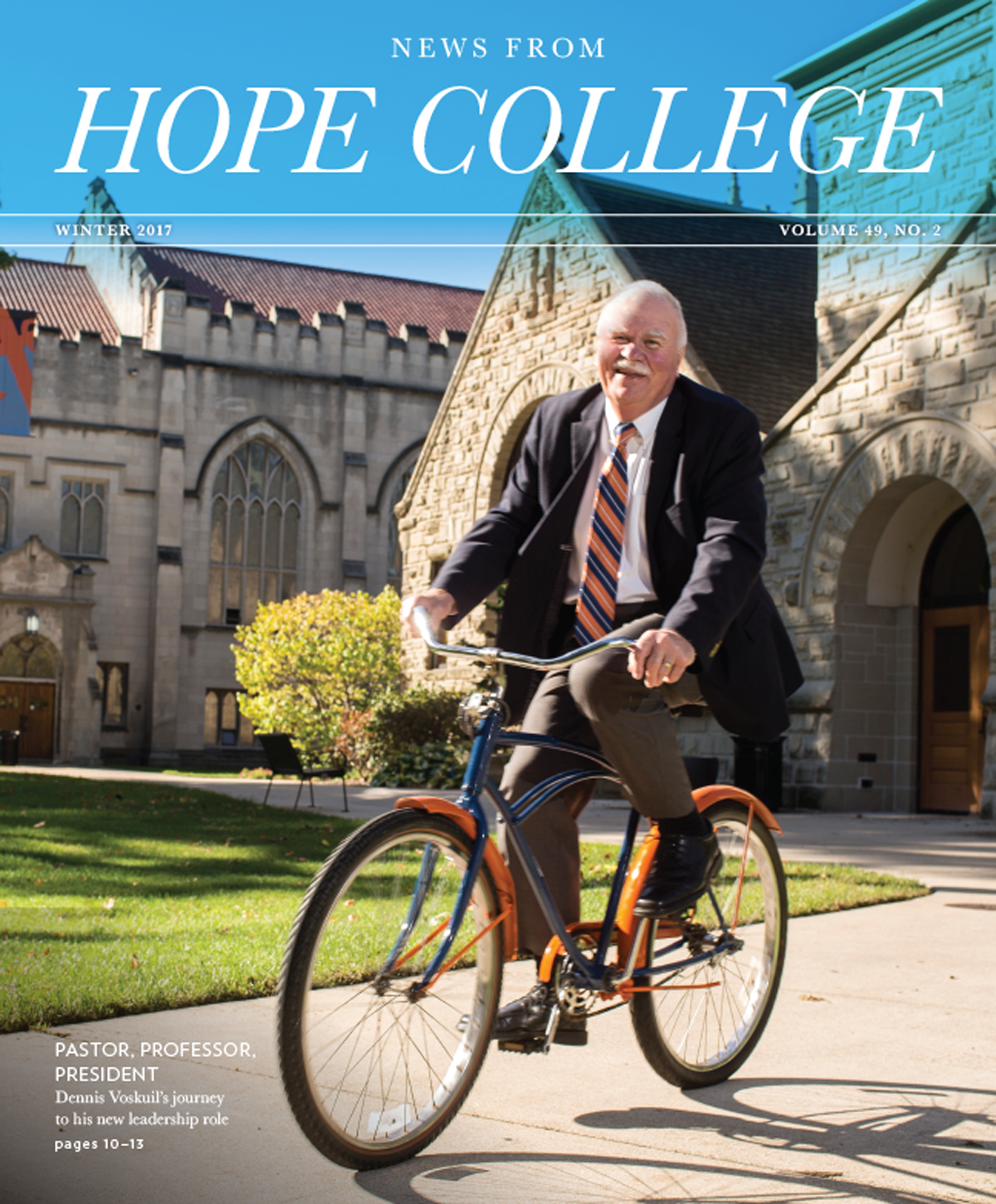Shifting Chemistry into Reverse
Jeffrey Johnson, Ph.D. | Professor of Chemistry
From the outside peering in, a great deal of organic chemistry looks like salt and water: white powders and transparent liquids.
Yet this branch of science is prolific in creating the things we use every day — toothbrushes, medications, milk jugs and other ubiquitous consumer products. The field has built these things so well, in fact, that many of them are incredibly hard-wearing (such as plastics that will hang around for centuries and drugs that cycle through waterways without completely breaking down). At the molecular level, the credit goes to the stability of the carbon-to-carbon bonds that hold these things together.
Usually, “organic chemistry is concerned with building these bonds — not taking them apart,” notes Dr. Jeff Johnson. But his Hope College research group is running in the opposite direction.
“The research that we do in the lab asks the question: How do we take carbon-carbon bonds and split them apart?” Johnson says. “Breaking carbon-carbon bonds is very, very difficult to do in any controlled fashion. And even when you can do it, turning that molecule into something else that could also be useful is incredibly challenging.” If this bond-breaking were to become possible, however, useful products would almost certainly result.
Johnson likens carbon-carbon bonds to the solid structure of a cinder block room. “The framework of a molecule is made from carbon-carbon bonds,” he says. “It’s like an elementary school gymnasium, in that it’s really easy to change the surface: You can put up posters, and you can paint it different colors. But ultimately, the walls of the gymnasium are still the same. What happens if you want to expand the gymnasium and make room for a second basketball court? You’d have to knock down a wall and push it out — and it’s almost impossible to do that without just building it from scratch. Essentially, organic synthesis is like that.”
Johnson and his student research assistants are in search of methods that would allow them to breakdown the walls efficiently. “What our method would do is allow us to selectively take some cinder blocks out and then put in a window or that second court,” Johnson says.
But why go to all this effort, simply to break something down? At the microscale, the breakdown of products can be just as creative a process as their building-up. A chemist who breaks a piece off a molecule is left with two different molecules — and each one could be valuable.
“We’re not looking to make a specific product or a specific pharmaceutical,” Johnson says. “We’re looking to make a tool that other chemists can use as they’d like.” Such a tool would allow scientists to rearrange molecules, chopping off a piece here and there to liberate completely new products.
Johnson sees a particular value for this method in the realm of drug development.
Currently, if a molecule proves effective against a cancerous cell line, it’s not always clear which part is actively achieving that effect. “For a complex molecule, we have to make the right-hand side, and we have to make the left-hand side, and test each of them separately, which takes a lot of steps,” Johnson says. The alternative approach he envisions would be to simply chop the molecule apart and test each piece for the desired property. Technology that would allow chemists to selectively extract pieces of larger compounds would allow the examination of many more molecules in a shorter timescale, potentially increasing the rate of pharmaceutical discovery.
In the summer of 2019, Johnson’s team made substantial headway toward their goals of both measuring the rate of breaking those carbon-carbon bonds and developing new ways to do it. It’s on these goals that Johnson and his team are focusing in this research supported by a three-year, $275,855 grant from the National Science Foundation.
“Typically,” Johnson says, “the activation process goes through a certain pathway. Because we now know how that pathway works, we can figure out how to stop the pathway partway through and create a new product — or to get it to react with something else, and go in a different direction.” On a relatively small scale, this is already happening.
“We’ve proven to ourselves that we can do this,” Johnson says. “But now, instead of getting 10 percent of the material to make the new product, how do we get 90 percent?” The complexity of developing new reactions that work with a wide variety of compounds is a perennial challenge for organic chemists. Making it all the harder is the fact that Johnson and his student research assistants can’t visually observe these reactions, so their experimental process must rely on other methods of scientific assessment.
“That’s always a challenge, particularly for beginning students,” Johnson says. “The vast majority of things in organic chemistry are colorless liquids and white solids, and we often work with very small amounts. Ultimately, it’s getting students to trust things besides their eyesight.” It’s about teaching — and learning —new methods of observation, including the use of complex instruments that use infrared, radio waves or electrical current to provide information about unseen molecules.
“One of the major things that you learn as a research scientist is that it very, very, very rarely works how you expect it to,” Johnson says. “But when you’re pushing the envelope, you need to keep your eyes open and your head up. A heckuva lot of failure goes into every success.”