Hope Goes Viral
Benjamin Kopek, Ph.D. | Assistant Professor of Biology
While the fight against viral and bacterial human pathogens stretches back to the dawn of human history, on some fronts we have yet to mount an effective defense. At the nanoscopic level, viruses have been infiltrating and using our cells with impunity for as long as they and we have existed. The battle against pathogenic bacteria that can sicken or kill humans turned the corner in 1948 with the isolation of the first broad-spectrum antibiotic, so named because of its effectiveness against bacteria of both broad classes (gram-positive and gram-negative). But broad-spectrum anti-virals have lagged behind; there are none on the market.
Yet.
Molecular biologist Dr. Benjamin Kopek is working to change that.
“The goal of our work is to eliminate or alleviate human suffering due to viral disease,” Kopek says.
A key to achieving that giant goal may lie in a tiny virus known as Flock House Virus (FHV).
“It’s one of the simplest models in the RNA class of viruses,” Kopek explains. “It works well for our research because it doesn’t infect humans.” It also works well because it nevertheless belongs to the same class of viruses that constitute the majority of human, animal and plant pathogens: positive-strand RNA viruses.
Viruses are typically more difficult to develop defenses against than other pathogens, because they’re insidious in a way that the others are not. Once a virus gains access to a host cell, it hijacks that cell’s replication machinery to reproduce the virus’s own genetic material. Viruses become so inextricably linked to the cells they’ve invaded that efforts to disable them are liable to harm the host cells as well.
By studying how FHV behaves once it has invaded the cells of fruit flies (which the virus is able to infect and kill), Kopek hopes to discover how FHV replicates itself, what elements of the host cell it depends on for that replication, and how the viral reproduction might be stopped.
This could provide clues about how to stop other positive-strand RNA viruses that share FHV’s replication method. The positive-strand RNA class includes such notorious pathogens as the current 2019 novel coronavirus, hepatitis C, West Nile virus, Zika and yellow fever — the last of which continues to kill 10,000 people each year, even though a yellow fever vaccine was introduced more than 80 years ago.
“What we’re mostly trying to understand is how it replicates the genome — how it makes more copies of its RNA,” Kopek says.
He and students in his Hope College research group know where replication takes place: in the cellular membrane. (If you recall high school biology class, you may also know it as the “phospholipid bilayer.”) Their research focuses on the lipid aspect of the membrane— the hydrocarbon molecules that are one of the membranes’ building blocks — because previous studies have shown certain lipids to be key components in viral replication.
“We can label the lipids and look at where they are relative to the virus replication factories,” Kopek explains. (His research group labels lipids by attaching fluorescent dyes, and then scrutinizes them under microscopes.) “We can knock out cellular genes using CRISPR to see how that affects virus replication.” Change to the cell’s environment or genetics is observed through the lens of how the virus (and its replication) responds. Determining how FHV replicates in various conditions could lead to better understanding of its pathogenic cousins.
“If we can find a common way that they all use to replicate, then we can potentially develop a drug against that common target,” Kopek says. A drug against a target common to Zika, yellow fever, West Nile virus and hepatitis C — and indefinite numbers of other emerging and unknown viruses — is patently appealing.
Kopek is a Towsley Research Scholar, selected for a four-year Hope College program that provides funding and dedicated research time to promising researchers on Hope’s faculty early in their careers. He’s keen to support basic science around the world; it is, after all, where vaccines and pharmaceuticals get their start. In that vein, a second major aim he pursues is making research more widely available.
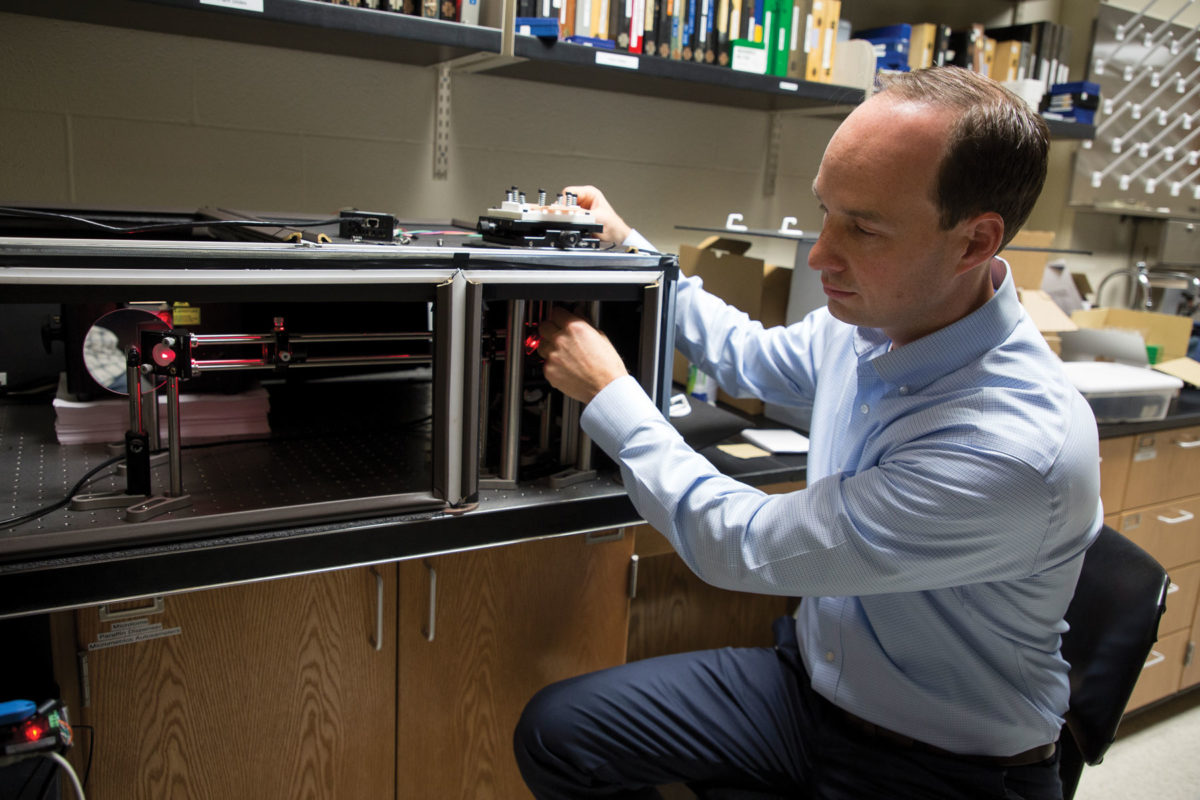
Assisted by Hope engineering graduate Justin Hanselman ’17, Kopek built the super-resolution fluorescence microscope that he uses for his research. As part of the growing open hardware movement, the two also developed a parts list and step-by-step assembly instructions — so other institutions could build for $10,000 to $15,000 a device that, produced commercially, would cost hundreds of thousands of dollars.
“It’s hopefully for the whole world,” he says. “That may not just be underdeveloped nations, but also undergraduate institutions or other specific labs that might not have a whole lot of funding, but have a need for these types of technologies.”
“The takeaway is that we need to support basic science research,” Kopek adds. “It informs our ability to treat human pathogens, and potentially deadly emerging pathogens, that could really be a threat to human health.” Likewise, anti-viral research must begin with the basics, uncovering the ways viruses operate in order to determine just how to stop them in their tracks.
“We need to have ways to understand the basics of this virus class, because there are more viruses out there than we’ll ever be able to develop vaccines against, or ever even know about,” Kopek says. “We need to do basic science research on this class of viruses so that we’re prepared.”